Are Soap A Compound . soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived from the fatty. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid.
from www.numerade.com
alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. The hydrophobic tail, derived from the fatty. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions.
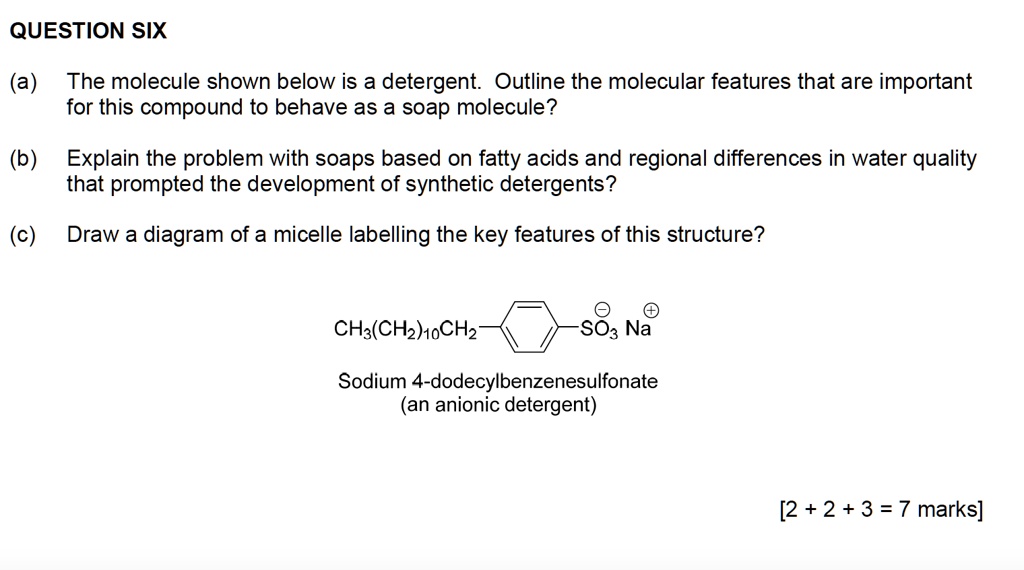
SOLVED QUESTION SIX (a) The molecule shown below is a detergent. Outline the molecular features
Are Soap A Compound soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. The hydrophobic tail, derived from the fatty. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of.
From www.youtube.com
How to make Soap Compound YouTube Are Soap A Compound soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived from the fatty. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the. Are Soap A Compound.
From www.youtube.com
CARBON AND COMPOUNDS 08 / SOAP PREPERATION / SAPONIFICATION / CLASS 10 / CHEMISTRY YouTube Are Soap A Compound soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also.. Are Soap A Compound.
From victoriaplum.com
How to make your own organic soap Are Soap A Compound soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. The hydrophobic tail, derived from the fatty. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with. Are Soap A Compound.
From homedecorjunky.blogspot.com
Soap Organic Compound Sodium Stearate Molecule Sodium Stearate Molecular Model Organic Are Soap A Compound alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived from the fatty. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “. Are Soap A Compound.
From www.indiamart.com
Soap Fragrance Compound, Oil at Rs 700/kg in Ahmedabad ID 26403048797 Are Soap A Compound soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. alkali. Are Soap A Compound.
From www.youtube.com
Carbon and Its Compound 06 Soaps and Detergent Class 10 NCERT Udaan YouTube Are Soap A Compound The hydrophobic tail, derived from the fatty. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap is a chemical compound resulting from the. Are Soap A Compound.
From www.youtube.com
Carbon and its compound(part8)CBSECLASS10SOAPS AND DETERGENTSCLEANSING ACTION OF SOAPS Are Soap A Compound soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. alkali metal salts. Are Soap A Compound.
From www.indiamart.com
Soap Compound at best price in Jaipur by Jugal Kishore Mehrotra Perfumers ID 4293970555 Are Soap A Compound alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap. Are Soap A Compound.
From www.alamy.com
A typical representation of a soap, a compound of one or more of the acids obtained from fatty Are Soap A Compound soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived. Are Soap A Compound.
From www.youtube.com
CLEANING ACTION OF SOAP CARBON AND ITS COMPOUND CLASS 10 YouTube Are Soap A Compound soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. alkali metal salts of fatty acids are more soluble in water than the acids. Are Soap A Compound.
From www.toppr.com
15. A chemical compound x is used in glass and soap industry. Identify the compound and give its Are Soap A Compound soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. The hydrophobic tail, derived from the fatty. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. soap molecules are amphiphilic, meaning they have. Are Soap A Compound.
From www.aliexpress.com
JX LCLYL Metal Brass Grinding Abrasive Buffing Polishing Soap Compound Paste Wax Bar Newin Wax Are Soap A Compound alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap is. Are Soap A Compound.
From stock.adobe.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK. Structural chemical formula Are Soap A Compound soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. alkali metal salts of fatty acids are more soluble in water than the. Are Soap A Compound.
From www.scribd.com
Soap Soap Chemical Substances Are Soap A Compound soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. The hydrophobic tail, derived from the fatty. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with. Are Soap A Compound.
From www.silverpolisher.com
Case of Silver Soap burnishing compound, 4 gallons, 128 ounces. Are Soap A Compound alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. soap molecules are amphiphilic, meaning they have both hydrophobic and hydrophilic regions. soap is a. Are Soap A Compound.
From www.youtube.com
Soaps Preparation of soap Hard & Soft water Cleansing action of Soap Carbon & its Are Soap A Compound soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. alkali metal salts of fatty acids are more soluble in water than the acids. Are Soap A Compound.
From www.studypool.com
SOLUTION Soap cleaning compound and toilet preparation manufacturing Studypool Are Soap A Compound soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or potassium hydroxide) with a fatty acid. soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. alkali metal salts of fatty acids are more soluble in water than the acids. Are Soap A Compound.
From www.huffingtonpost.com
Soap Compound Could Make It Easier For Staph Bacteria To Colonize In Your Nose HuffPost Are Soap A Compound soap is a salt of an alkali metal, such as sodium or potassium, with a mixture of “ fatty ” carboxylic acids. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of. soap is a chemical compound resulting from the reaction of an alkali (commonly sodium or. Are Soap A Compound.