Fda Shipping Container Labeling . section 5(b) of the fair packaging and labeling act provides for the establishment by regulation of exemptions from certain. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. questions have been raised as to whether or not the fdc act requires mandatory label information on such. a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in the united states.
from randyfchannelzz.blogspot.com
the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. questions have been raised as to whether or not the fdc act requires mandatory label information on such. a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in the united states. section 5(b) of the fair packaging and labeling act provides for the establishment by regulation of exemptions from certain. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in.
Ucc 128 Label Template Https Cdn Ymaws Com Bisg Org Resource Resmgr Files Publications Labels
Fda Shipping Container Labeling a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. questions have been raised as to whether or not the fdc act requires mandatory label information on such. the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. section 5(b) of the fair packaging and labeling act provides for the establishment by regulation of exemptions from certain. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in the united states.
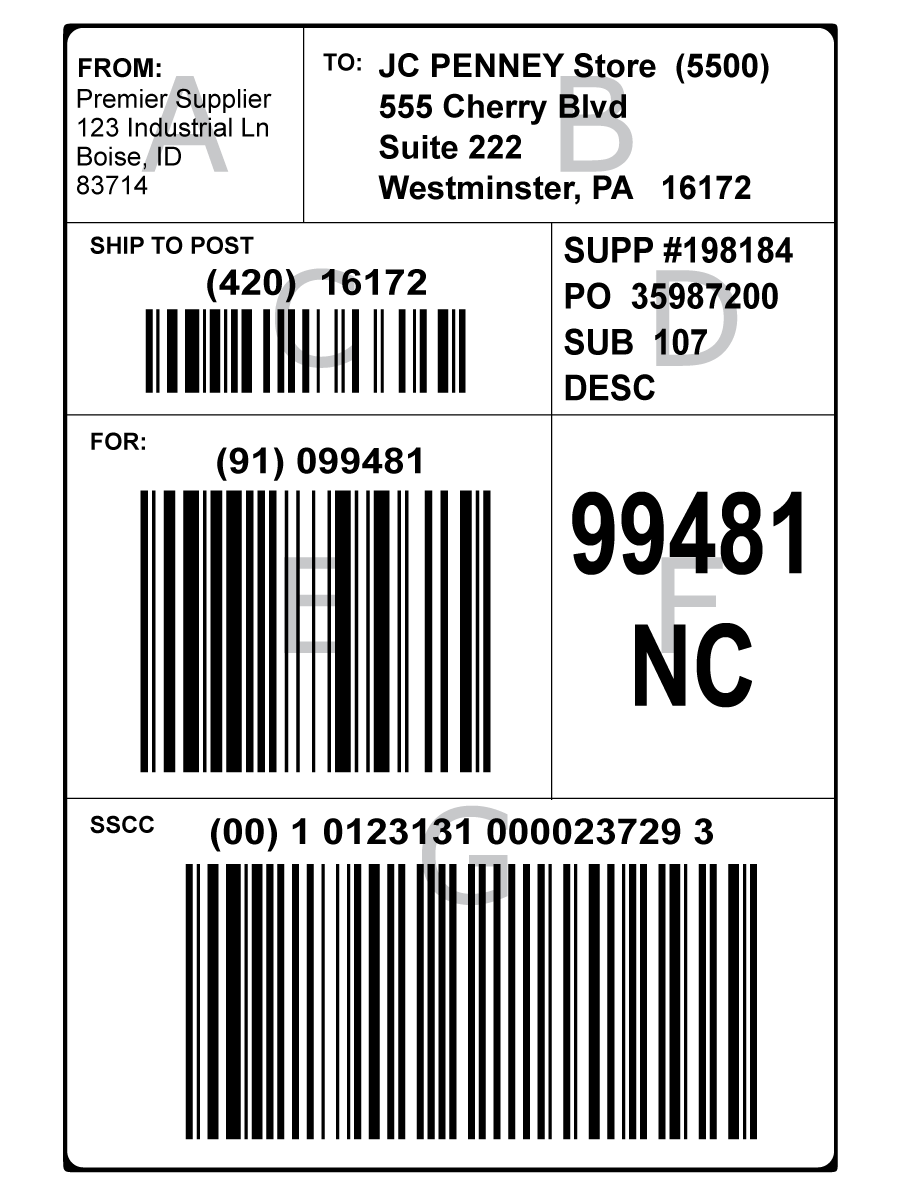
From ediacademy.com
Shipping Carton Labeling RequirementsEDI Blog EDI Blog Fda Shipping Container Labeling the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in. Fda Shipping Container Labeling.
From www.container-xchange.com
Shipping container doors Guide [+ buy units with lockbox] Fda Shipping Container Labeling a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. questions have been raised as to whether or not the fdc act requires mandatory label information on such. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in. Fda Shipping Container Labeling.
From www.promptus.us
Markings on a Shipping Container What do They Mean? Promptus LLC Fda Shipping Container Labeling questions have been raised as to whether or not the fdc act requires mandatory label information on such. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in the. Fda Shipping Container Labeling.
From dandkmotorsports.com
Osha Regulations For Spray Paint Storage Dandk Organizer Fda Shipping Container Labeling the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. questions have been raised as to whether or not the fdc act requires mandatory label information on such. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in. Fda Shipping Container Labeling.
From www.slideserve.com
PPT Hazard Communication PowerPoint Presentation, free download ID6813642 Fda Shipping Container Labeling a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. . Fda Shipping Container Labeling.
From ediacademy.com
Shipping Label Requirements (Burlington)EDI Blog EDI Blog Fda Shipping Container Labeling section 5(b) of the fair packaging and labeling act provides for the establishment by regulation of exemptions from certain. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in the united states. the controlled substances act requires a symbol to identify the schedule of each controlled substance. Fda Shipping Container Labeling.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Fda Shipping Container Labeling the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. . Fda Shipping Container Labeling.
From printable.conaresvirtual.edu.sv
Free Printable Osha Secondary Container Label Template Fda Shipping Container Labeling questions have been raised as to whether or not the fdc act requires mandatory label information on such. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. a. Fda Shipping Container Labeling.
From randyfchannelzz.blogspot.com
Ucc 128 Label Template Https Cdn Ymaws Com Bisg Org Resource Resmgr Files Publications Labels Fda Shipping Container Labeling (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. questions have been raised as to whether or not the fdc act requires mandatory label information on such. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in the. Fda Shipping Container Labeling.
From www.ehs.washington.edu
Chemical Container Labels EHS Fda Shipping Container Labeling section 5(b) of the fair packaging and labeling act provides for the establishment by regulation of exemptions from certain. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. questions have been raised as to whether or not the fdc act requires mandatory label information on such. this. Fda Shipping Container Labeling.
From blog.globalvision.co
How to Master FDA Labeling Requirements Fda Shipping Container Labeling a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. section 5(b) of the fair packaging and labeling act provides for the establishment by regulation of exemptions from certain. . Fda Shipping Container Labeling.
From ehs-web01.s.uw.edu
Chemical Container Labels EHS Fda Shipping Container Labeling (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. . Fda Shipping Container Labeling.
From old.sermitsiaq.ag
Printable Secondary Container Labels Fda Shipping Container Labeling this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in the united states. a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. the controlled substances act requires a symbol to identify the schedule of each controlled substance. Fda Shipping Container Labeling.
From www.drake.edu
Container Labels Drake University Fda Shipping Container Labeling questions have been raised as to whether or not the fdc act requires mandatory label information on such. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. section 5(b) of the fair packaging and labeling act provides for the establishment by regulation of exemptions from certain. a. Fda Shipping Container Labeling.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Shipping Container Labeling questions have been raised as to whether or not the fdc act requires mandatory label information on such. a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. . Fda Shipping Container Labeling.
From blog.containers.guide
Shipping Container Labeling. Going through ISO — Containers.Guide Fda Shipping Container Labeling the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. questions have been raised as to whether or not the fdc act requires mandatory label information on such. section. Fda Shipping Container Labeling.
From blog.containers.guide
Shipping Container Labeling. Going through ISO — Containers.Guide Fda Shipping Container Labeling the controlled substances act requires a symbol to identify the schedule of each controlled substance on the label or. this web page contains the official text of 21 cfr part 801, which regulates the labeling of medical devices in the united states. (a) shipping containers or wrappings used solely for the transportation of any such commodity in. Fda Shipping Container Labeling.
From blog.containers.guide
Shipping Container Labeling. Going through ISO — Containers.Guide Fda Shipping Container Labeling section 5(b) of the fair packaging and labeling act provides for the establishment by regulation of exemptions from certain. a package or market package 7 refers to the container closure system and labeling, associated components (e.g., dosing cups,. (a) shipping containers or wrappings used solely for the transportation of any such commodity in bulk or in. . Fda Shipping Container Labeling.